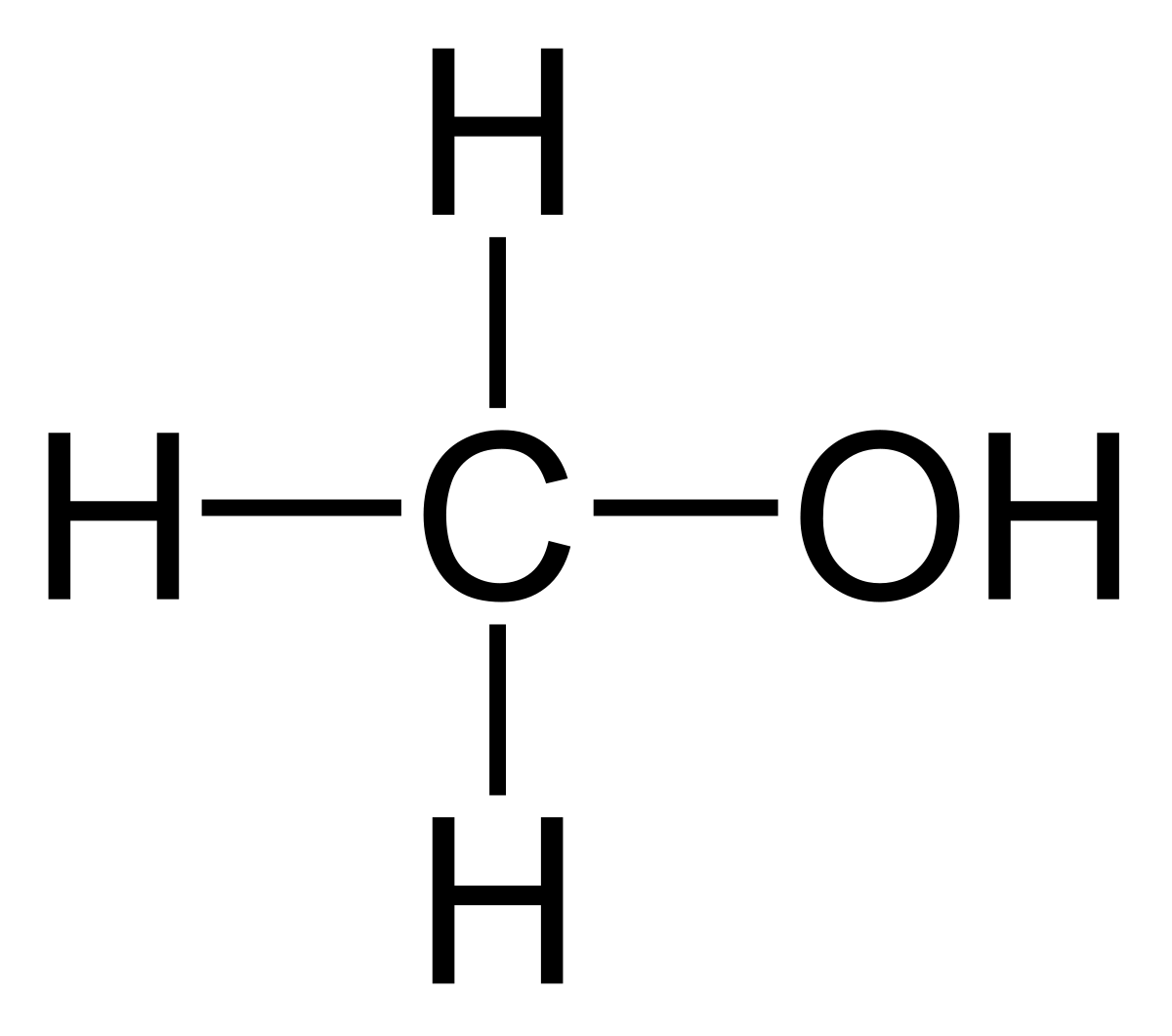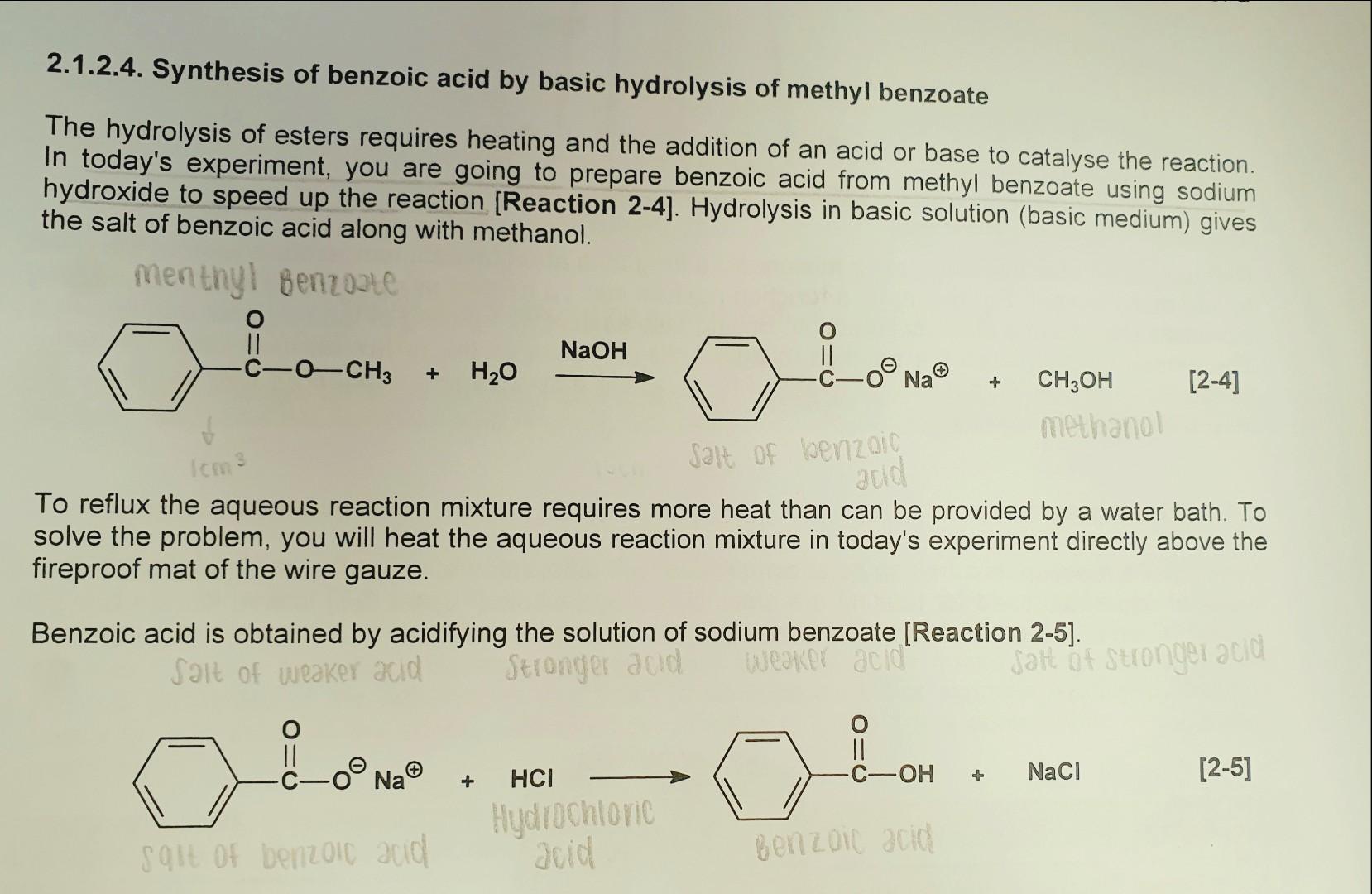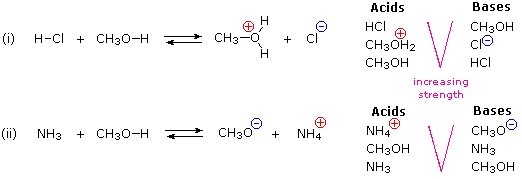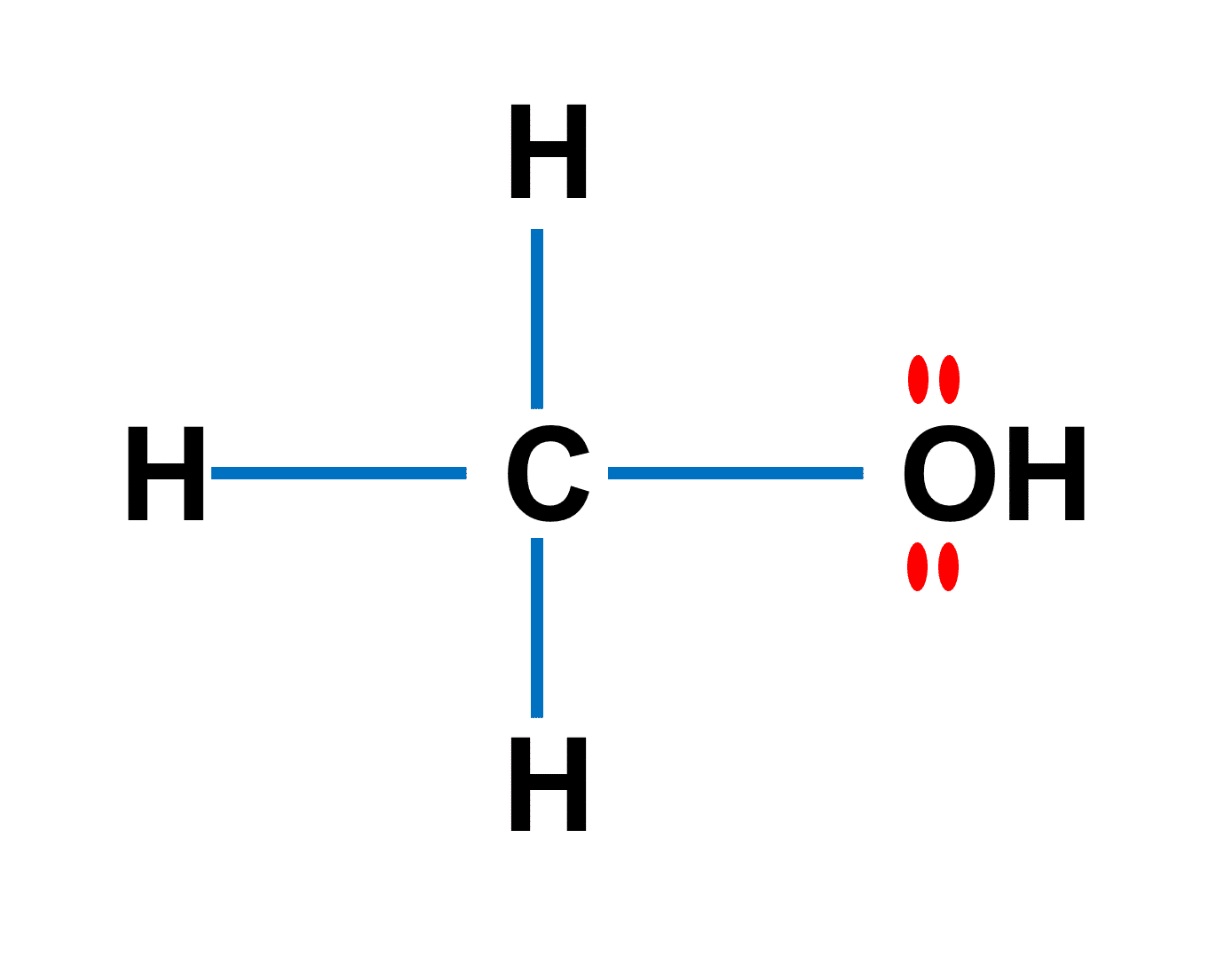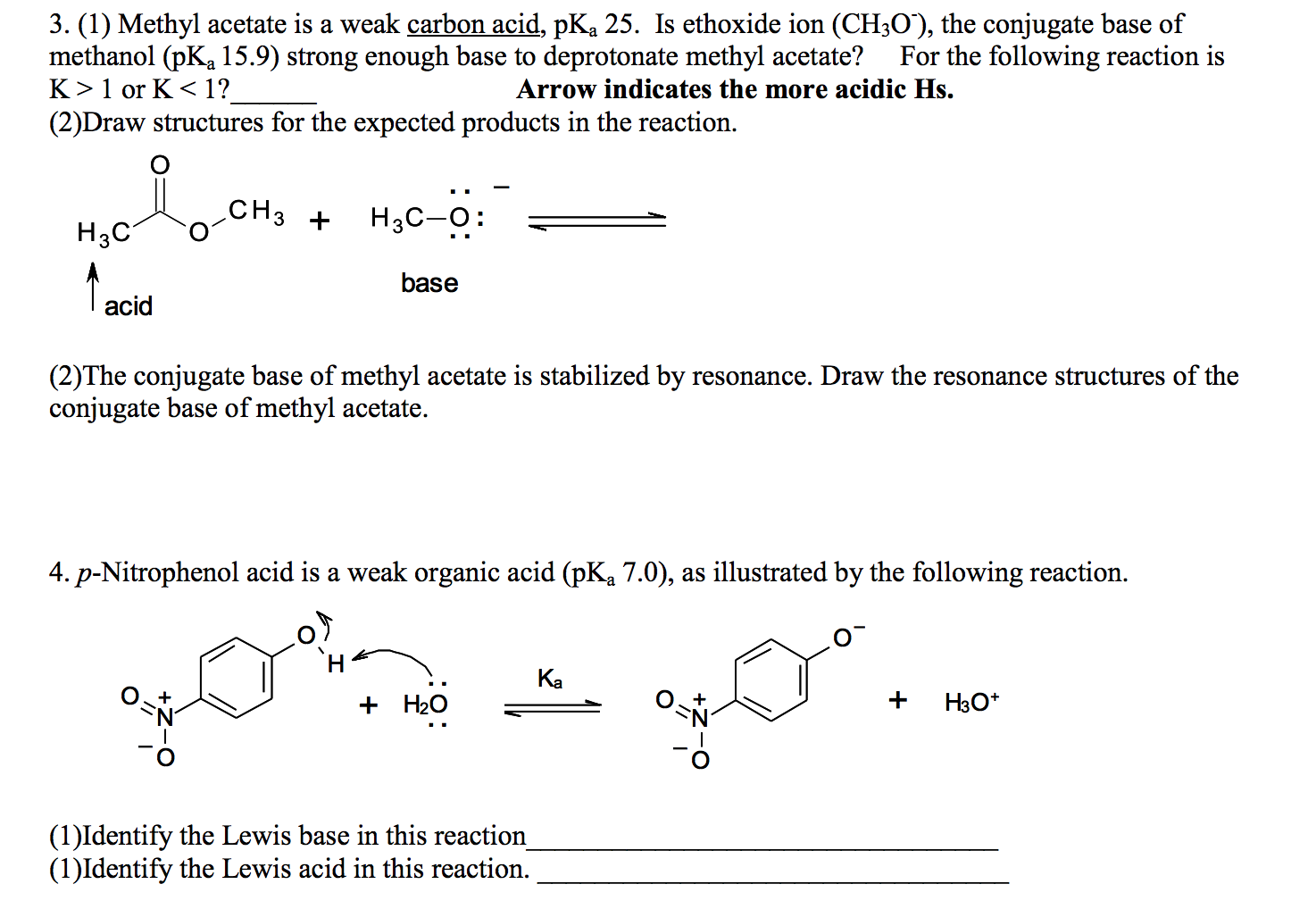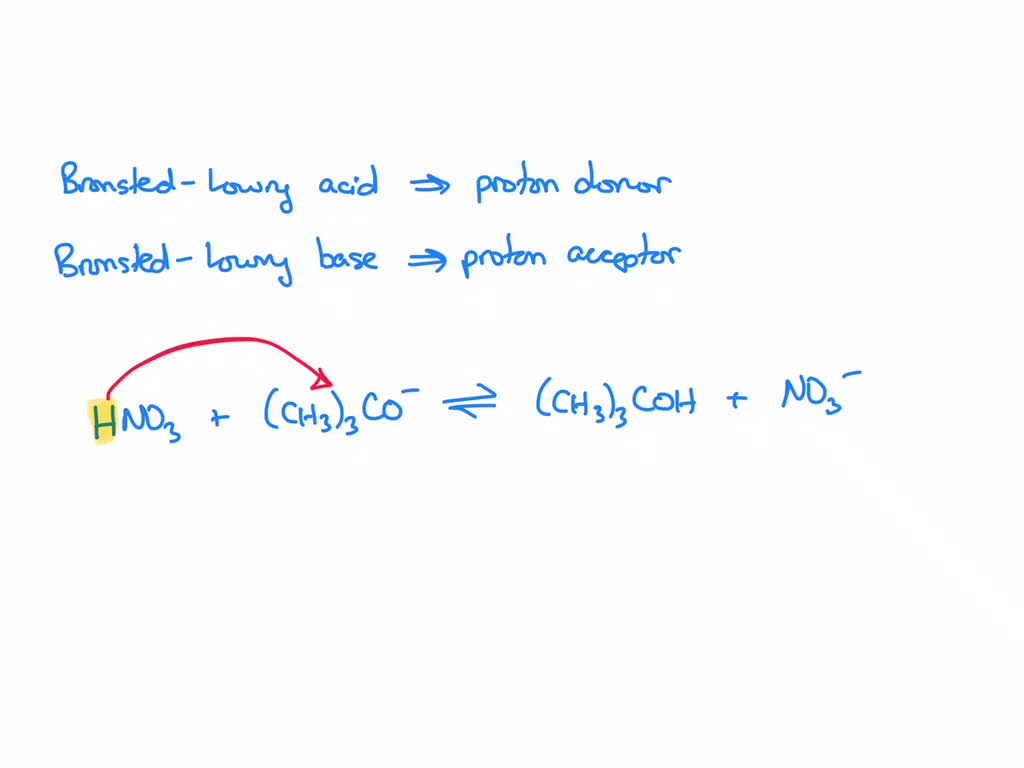
SOLVED: Label each reactant and product in this reaction as a bronsted acid or base. CH3OH +OH- <—> CH3O- +H2O
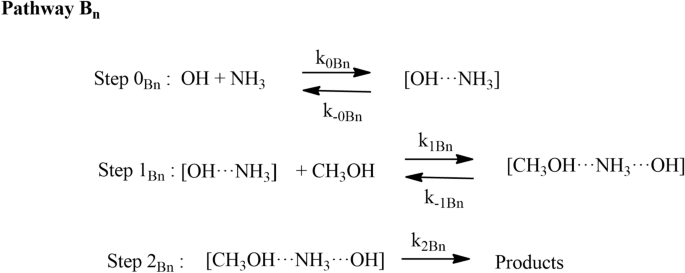
Effect of ammonia and water molecule on OH + CH3OH reaction under tropospheric condition | Scientific Reports
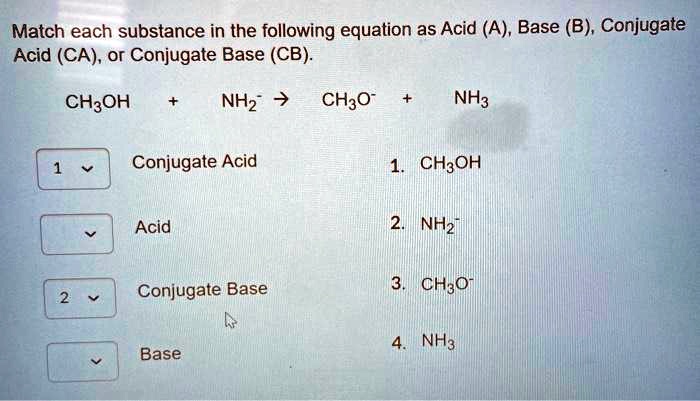
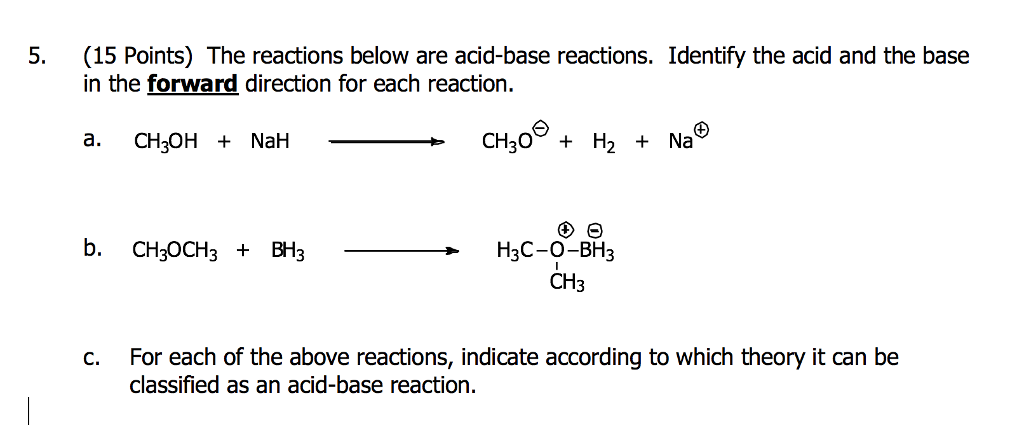
SOLVED: Match each substance in the following equation as Acid (A), Base (B), Conjugate Acid (CA), or Conjugate Base (CB) CH3OH NH2 CH3O" NH3 Conjugate Acid CHzOH Acid NH2 Conjugate Base CH3o-

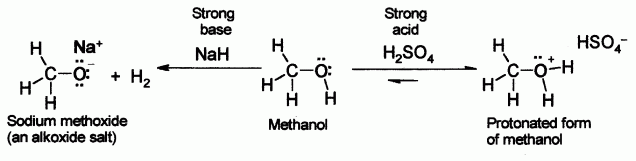
SOLVED:Alcohols can act either as weak acids or as weak bases, just as water can. Show the reaction of methanol, CH3 OH, with a strong acid such as HCl and with a

Delocalization state-induced selective bond breaking for efficient methanol electrosynthesis from CO2 | Nature Catalysis