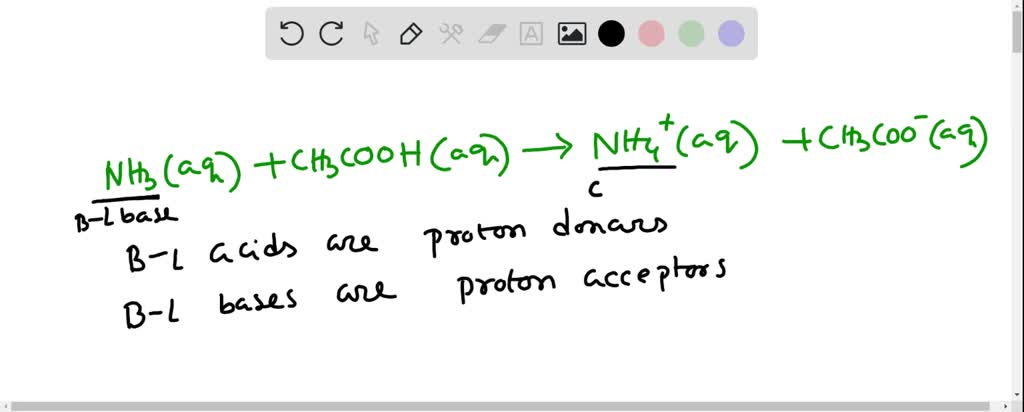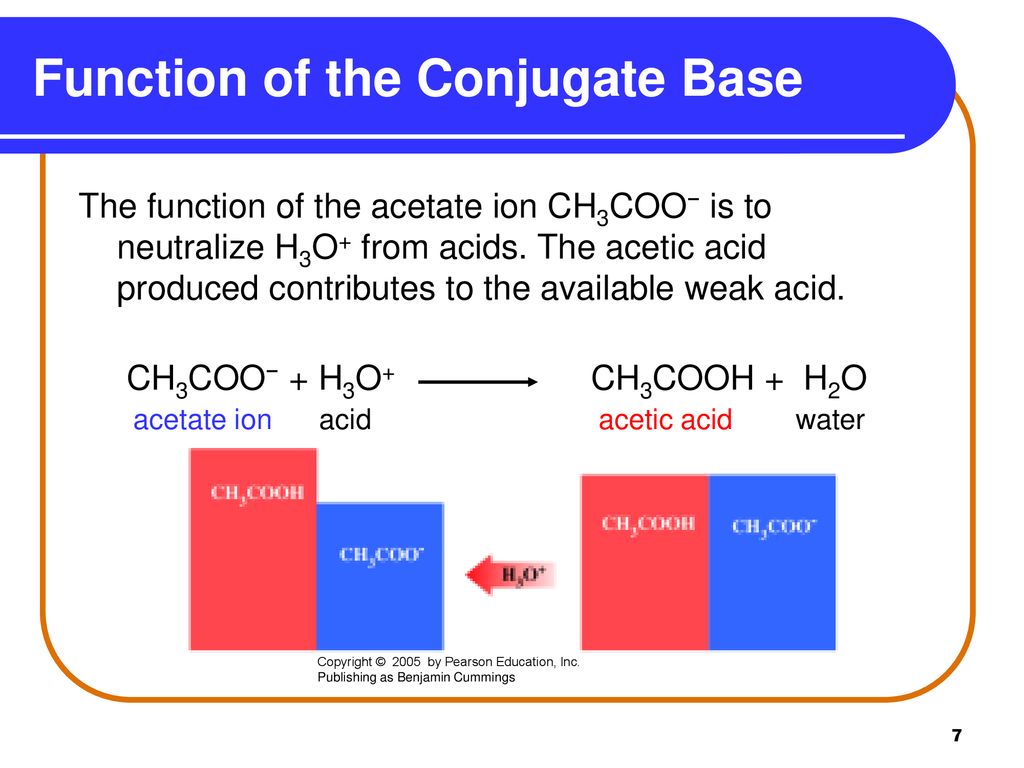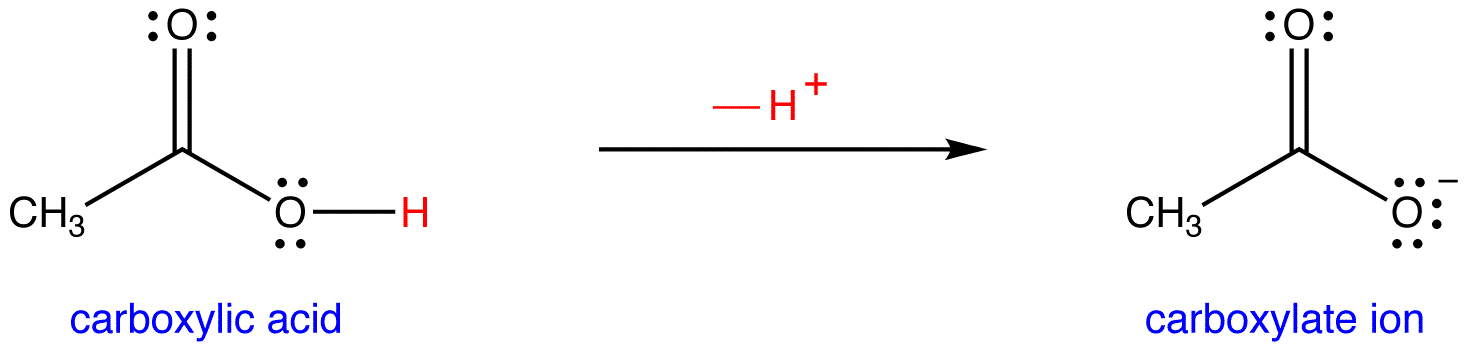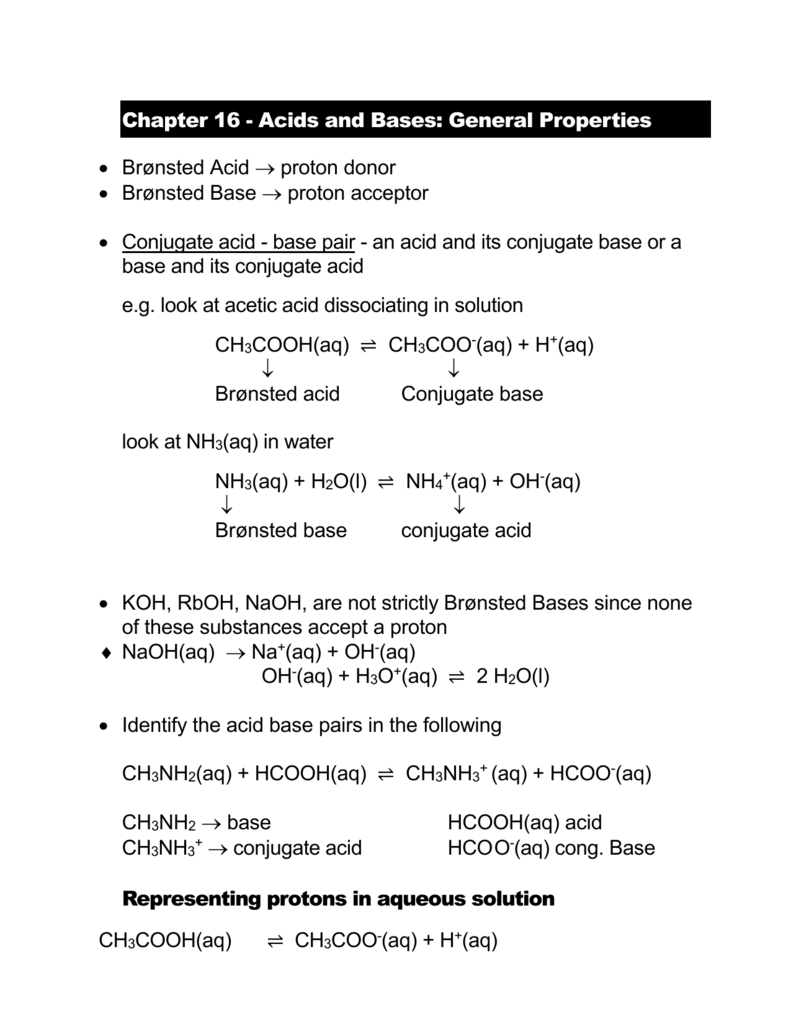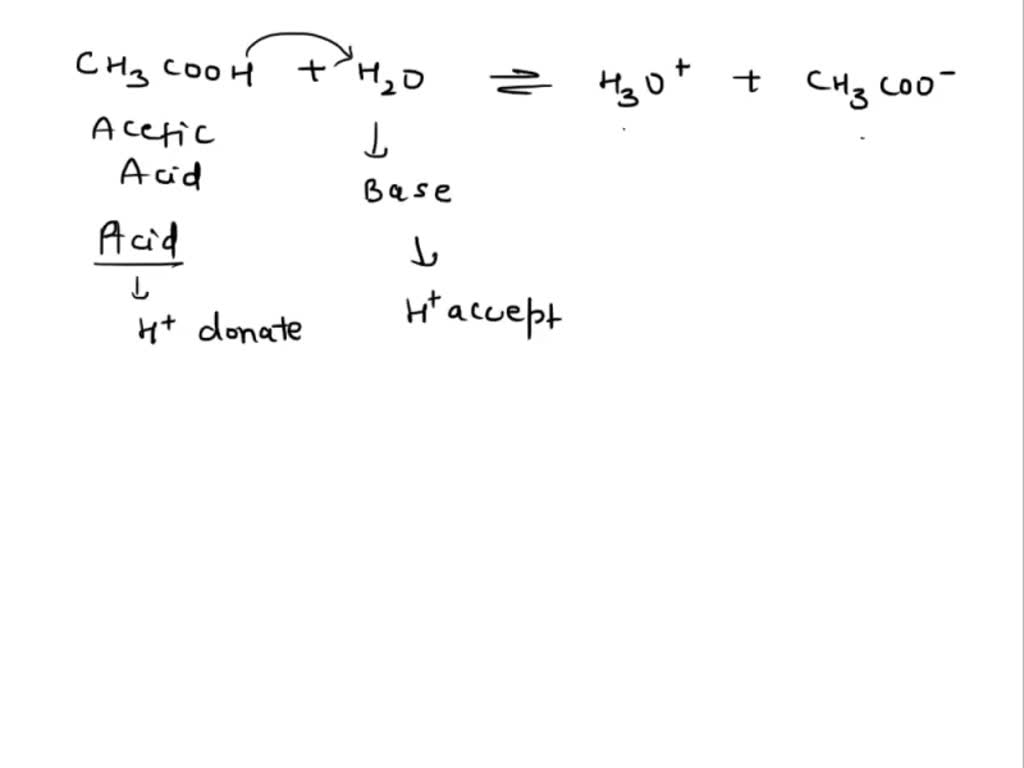
SOLVED: Question 7 In in the dissolution of acetic acid in water; CH3COOH H2o = HaOt + CH3COO" CH3COOH is the base and CH3COO- is the conjugate acid CH3COOH is the base

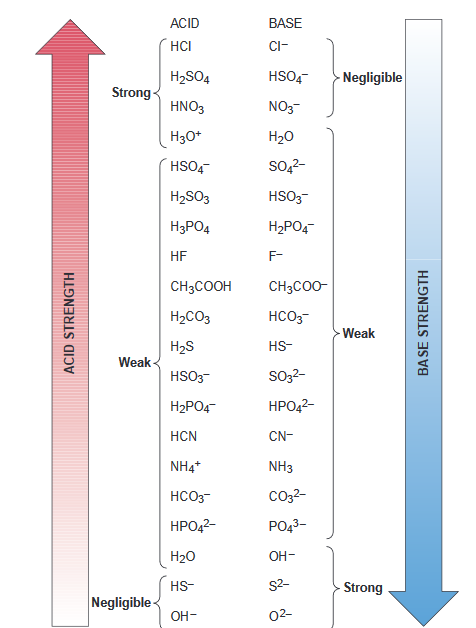
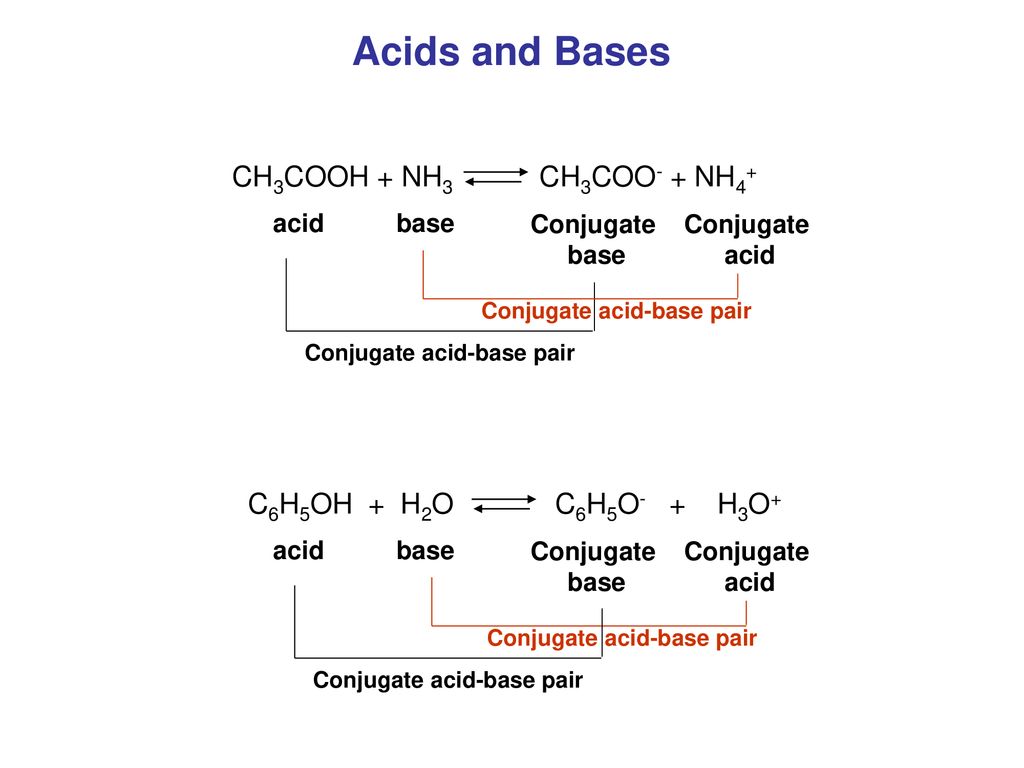
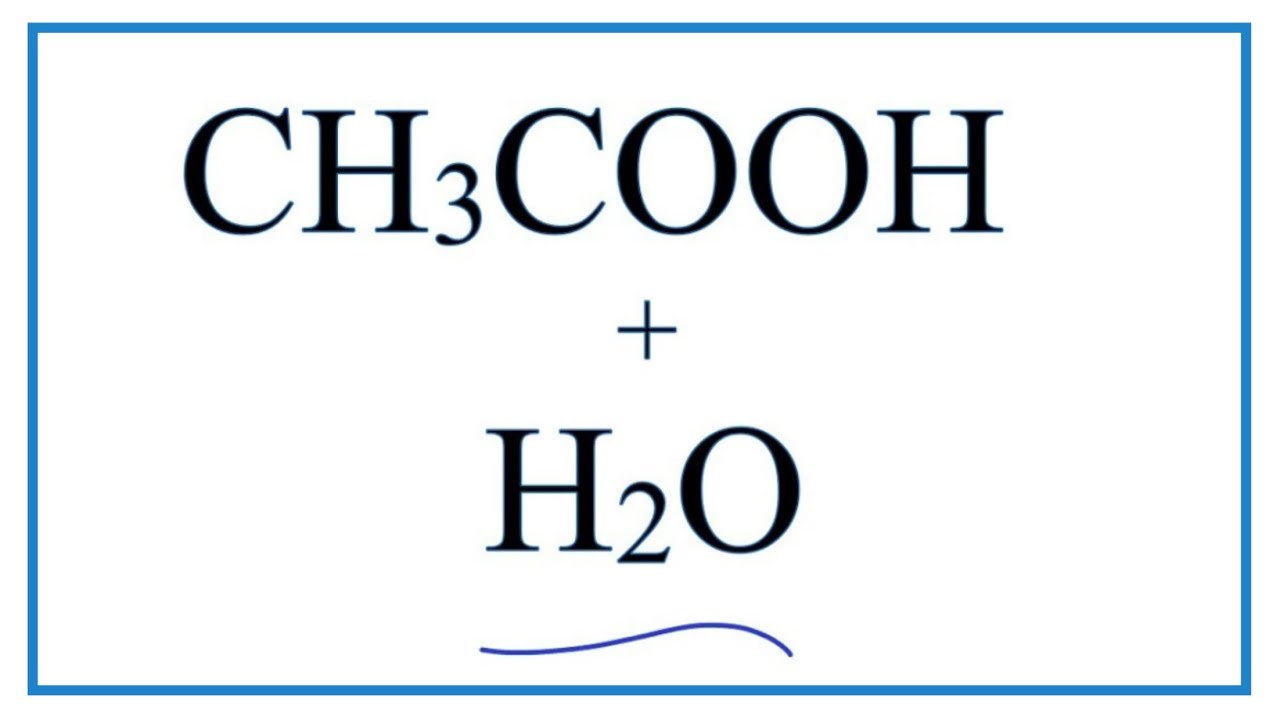
Ionic dissociation of acetic acid is represented as CH3COOH + H2O iff H3O^(+) + CH3COO^- Which of the following statement are/is correct? 1. According to Lowery and Bronsted, the reaction posses an
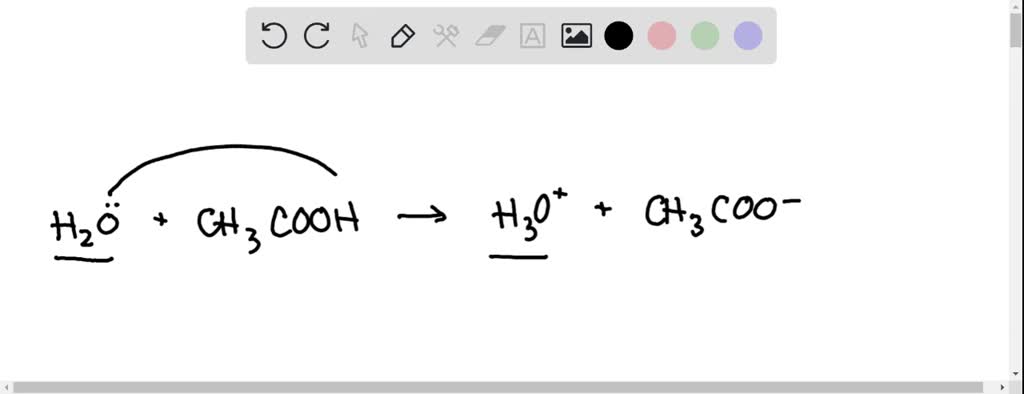
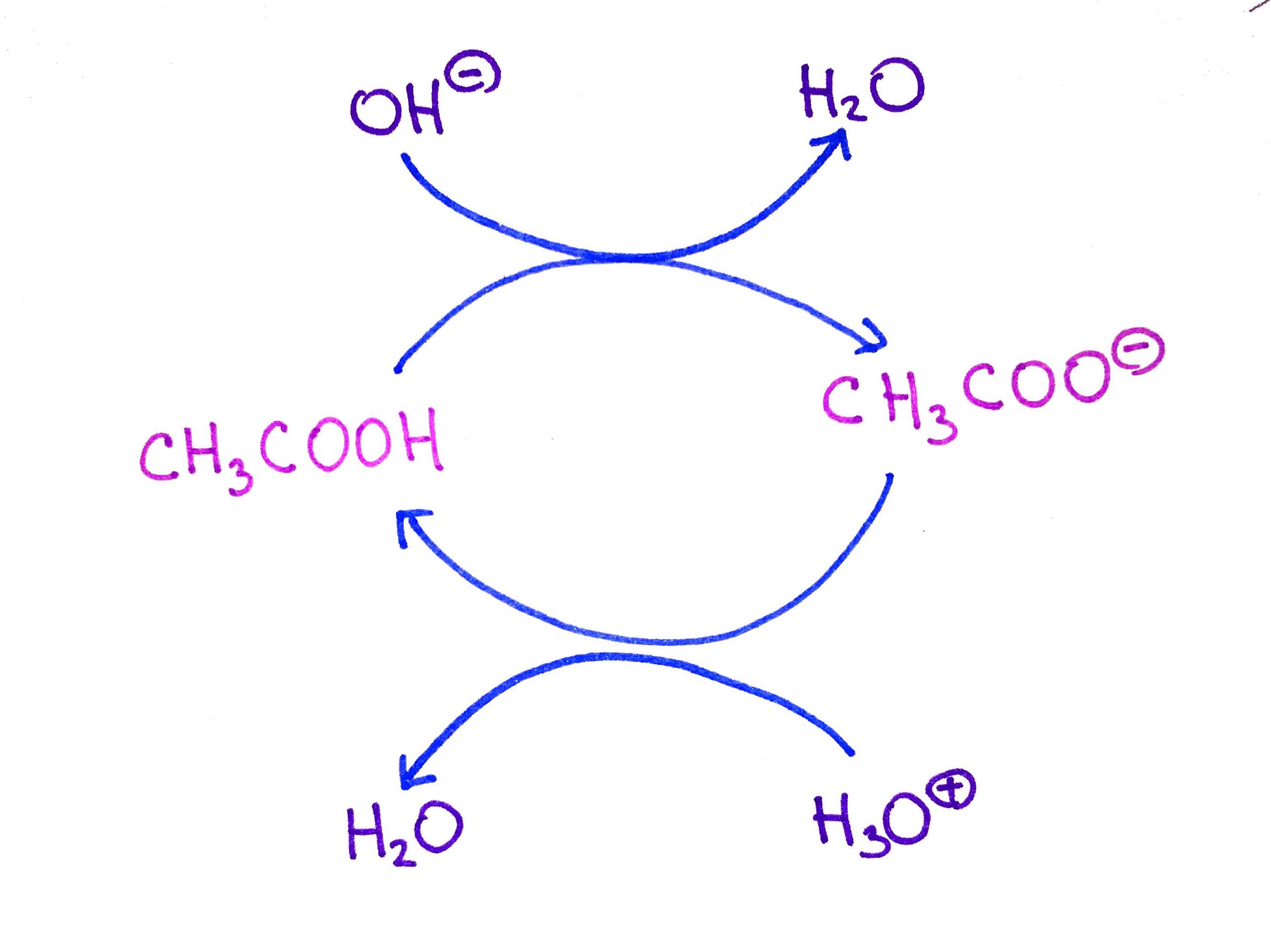
SOLVED: In the following chemical equation, identify the Bronsted-Lowry acid and the Bronsted-Lowry base H2O + CH3COOH CH3COO− + H3O+